Introduction
The medical device industry operates in an environment where quality, safety, and reliability are non-negotiable. Even minor product failures can lead to serious patient harm, regulatory action, and reputational damage. As healthcare systems evolve and regulations tighten worldwide, medical device organizations are expected to demonstrate consistent control over their processes and products. This growing focus on regulatory compliance has made structured quality management systems essential rather than optional.
The ISO 13485 standard has emerged as a globally recognized benchmark specifically designed for medical devices. Unlike general quality frameworks, it addresses the unique regulatory, risk, and lifecycle requirements of healthcare products. From design and development to distribution and post-market activities, ISO 13485 medical devices compliance helps organizations align with international expectations. As a result, manufacturers, suppliers, and service providers increasingly rely on this standard to ensure safety, build trust, and achieve global market acceptance.
1. What Is ISO 13485?
ISO 13485 is an international quality management system standard developed specifically for the medical device industry. It defines the requirements organizations must follow to consistently design, manufacture, install, and service medical devices that meet both customer expectations and regulatory obligations. The primary purpose of the ISO 13485 standard is to ensure that patient safety, product effectiveness, and regulatory compliance are embedded throughout the entire medical device lifecycle.
The scope of ISO 13485 medical devices compliance extends beyond manufacturers. It also applies to component suppliers, distributors, contract manufacturers, and service providers involved in healthcare products. What makes ISO 13485 unique is its sector-specific focus. Rather than emphasizing broad business efficiency alone, it prioritizes risk management, documentation, traceability, and regulatory alignment. This targeted approach is why ISO 13485 is widely regarded as the most appropriate quality management framework for organizations operating within the medical device ecosystem.
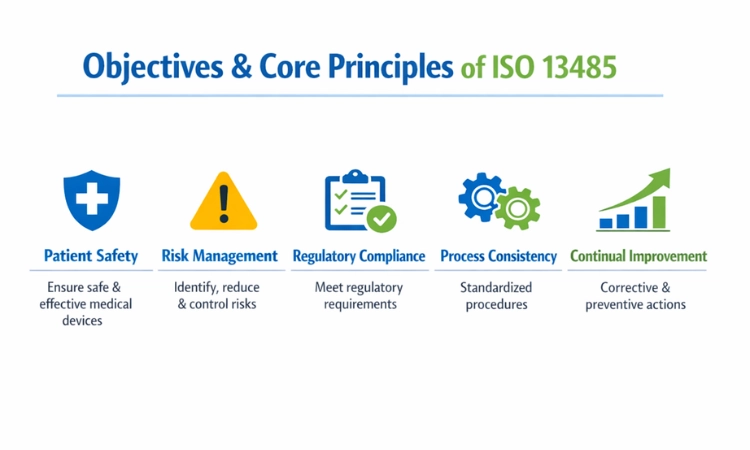
2. Objectives and Core Principles of ISO 13485
At its core, ISO 13485 is built around protecting patients while ensuring medical devices perform as intended. One of its primary objectives is to reduce risks associated with device design, production, and post-market use through a structured, risk-based approach. Risk management is not treated as a one-time activity but as a continuous process integrated across product realization.
Another key principle is strong regulatory compliance supported by controlled documentation and records. ISO 13485 requires organizations to maintain clear procedures, defined responsibilities, and full traceability of materials and processes. Consistency in operations is emphasized to minimize variability and errors. Additionally, the standard promotes a culture of continual improvement by encouraging corrective and preventive actions. Together, these principles help medical device companies achieve reliable outcomes while maintaining accountability and transparency throughout their quality management system.
3. Why ISO 13485 Is Important for Medical Device Companies
ISO 13485 plays a critical role in helping medical device companies deliver safe and effective products. By implementing ISO 13485 certified systems, organizations can systematically identify and control risks that may impact patient safety. This structured approach significantly reduces defects, product recalls, and regulatory non-compliance issues.
The standard also supports alignment with national and international regulatory expectations, making it easier for companies to operate across multiple markets. Hospitals, regulators, and healthcare providers increasingly prefer working with ISO 13485 certified companies because it demonstrates a commitment to quality and compliance. For startups and small manufacturers, certification builds early credibility, while established organizations benefit from enhanced operational discipline. In highly competitive healthcare markets, ISO 13485 certification provides a clear competitive advantage by reinforcing trust, reliability, and long-term sustainability.
4. ISO 13485 vs ISO 9001 Quality Management System
While both ISO 13485 and the ISO 9001 Quality Management System, Business framework focus on quality, their objectives and scope differ significantly. ISO 9001 is a generic standard applicable to any industry and emphasizes overall business efficiency and customer satisfaction.
In contrast, ISO 13485 is tailored specifically for medical devices, with a strong focus on regulatory compliance, risk management, and product safety. Documentation requirements under ISO 13485 are more rigorous, reflecting healthcare regulatory expectations. Many healthcare-related Business organizations choose to implement both standards, using ISO 9001 for general quality improvement and ISO 13485 for regulatory-driven medical device operations. This combined approach helps organizations maintain robust quality systems while meeting strict healthcare compliance requirements.
ISO 13485 vs ISO 9001: Key Differences
| Aspect | ISO 13485 Standard | ISO 9001 Quality Management System, Business |
| Industry Focus | Specifically for medical devices and healthcare-related products | Applicable to all industries and Business sectors |
| Primary Objective | Patient safety, product effectiveness, and regulatory compliance | Customer satisfaction and overall Business improvement |
| Regulatory Emphasis | Strong alignment with global medical device regulations | Minimal regulatory focus |
| Risk Management | Mandatory and integrated throughout product lifecycle | Risk-based thinking encouraged but less prescriptive |
| Documentation Requirements | Extensive and strictly controlled | Flexible documentation requirements |
| Design & Development Controls | Detailed and mandatory | Optional depending on Business scope |
| Market Expectation | Often required for medical device approval and market access | Rarely mandated by regulators |
| Typical Users | Medical device manufacturers, suppliers, service providers | Any Business seeking quality improvement |
5. Who Needs ISO 13485 Certification?
ISO 13485 certification is relevant to a wide range of organizations involved in the medical device supply chain. This includes medical device manufacturers, component and raw material suppliers, and contract manufacturing partners. Service providers such as sterilization companies, packaging firms, calibration services, and testing laboratories are also covered under the standard.
Additionally, distributors and organizations involved in installation, servicing, or maintenance of medical devices can benefit from ISO certification for company operations in healthcare. Any organization that directly or indirectly affects the safety, quality, or performance of medical devices is encouraged to adopt ISO 13485 to demonstrate compliance and accountability.
6. Key Requirements of ISO 13485 Standard
The ISO 13485 standard outlines comprehensive requirements designed to ensure consistent quality across medical device operations. A documented quality management system is mandatory, including policies, procedures, and records that support regulatory compliance. Risk management must be applied throughout the product lifecycle, from initial design to post-market activities.
Design and development controls ensure products meet intended use and safety requirements. Supplier and outsourcing controls are critical to maintaining quality across external partners. Production and monitoring processes emphasize traceability, cleanliness, and controlled environments where applicable. Additionally, organizations must implement effective complaint handling, corrective actions, and preventive measures. These requirements collectively ensure ISO 13485 medical devices remain safe, reliable, and compliant in real-world use.
7. Benefits of Being ISO 13485 Certified
Achieving ISO 13485 certified status delivers both regulatory and operational advantages. One of the most significant benefits is improved acceptance by regulatory authorities, reducing approval delays and compliance risks. Internally, organizations experience better process consistency, clearer responsibilities, and enhanced efficiency.
Certification also supports broader market access, enabling organizations to compete in domestic and international healthcare markets. Customers, partners, and stakeholders gain confidence when working with ISO 13485 certified companies, as it signals reliability and accountability. Over time, these benefits contribute to long-term Business sustainability and a stronger organizational reputation within the medical device industry.
8. Common Mistakes to Avoid During ISO Certification in India
Common Mistakes to Avoid During ISO Certification in India often stem from misunderstanding the purpose of the standard. Treating certification as a paperwork exercise rather than a functional system is a frequent issue. Poor implementation of risk management, inadequate employee training, and ignoring regulatory updates can weaken compliance efforts.
Organizations may also choose unrealistic certification timelines or fail to align ISO requirements with actual Business processes. These mistakes can lead to audit failures and ineffective quality systems. Avoiding them requires practical implementation, leadership involvement, and ongoing monitoring of regulatory expectations.
9. Challenges in Implementing ISO 13485 and How to Overcome Them
Implementing ISO 13485 can be challenging due to extensive documentation requirements and complex regulatory interpretations. Resource constraints and costs may also impact smaller organizations. Supplier compliance is another common difficulty, especially when managing multiple external partners.
These challenges can be addressed through structured planning, clear role allocation, and comprehensive employee training. Gradual implementation, internal audits, and continuous improvement help organizations overcome obstacles while maintaining long-term compliance and system effectiveness.
Importance of ISO Certification for Medical Device Organizations
ISO certification plays a vital role in safeguarding both patients and organizations. ISO 13485 certified systems help ensure consistent product quality, regulatory compliance, and operational discipline. By adopting the iso 13485 standard, iso 13485 certified companies demonstrate accountability across iso 13485 medical devices operations. This structured approach supports credibility, long-term compliance, and responsible growth within the healthcare ecosystem.
10. Conclusion
ISO 13485 has become a foundational requirement for medical device quality and safety. It provides a structured framework that aligns regulatory compliance with patient-centric product design and manufacturing. Beyond meeting regulatory expectations, the standard supports long-term Business resilience, operational consistency, and global market access.
In increasingly competitive healthcare environments, ISO 13485 is no longer optional, highlighting the importance of ISO certification for organizations operating in regulated healthcare markets. Organizations that adopt and maintain this standard position themselves as reliable, compliant, and quality-driven partners. By embedding risk management, documentation, and continuous improvement into daily operations, medical device companies can meet evolving regulatory demands while protecting patients and sustaining trust.
Medical device organizations are encouraged to evaluate their current quality management maturity and alignment with ISO 13485 requirements. Conducting internal audits, strengthening employee awareness, and integrating regulatory expectations into daily operations can improve readiness. A structured, informed approach to ISO frameworks supports compliance, consistency, and long-term organizational confidence.